Potential Energy Surface of the Water Molecule¶
Overview¶
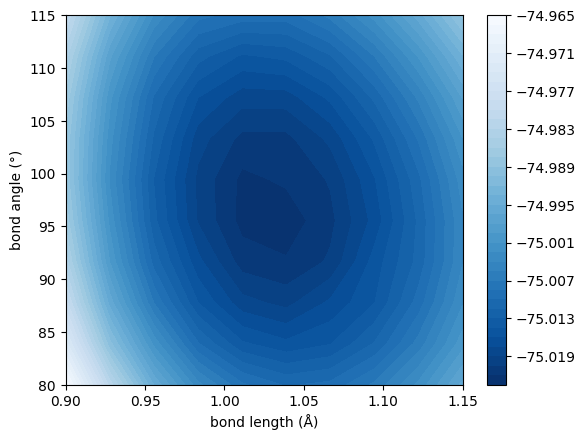
In this notebook, we will use TenCirChem to build the potential energy surface of \(\rm{H_2O}\) molecule at UCCSD/STO-3G level of theory. Without incurring active space approximation, the system consists of 6 spatial orbitals and 8 electrons. Under Jordan-Wigner approximation, 12 qubits are required to simulate the system.
We highlight how easy it is to solve chemistry problems with TenCirChem. We also highlight that the whole PES consisting of 100 points is contructed on a laptop within 1 minute.
Setup¶
Build a series of PySCF moles for UCCSD calculation.
The bond angles range from 80° to 115° and the bond lengths range from 0.90Å to 1.15Å. 10 points are sample from each DOF, resulting in totally 100 geometries.
[1]:
import numpy as np
# TenCirChem accepts PySCF Mole as input
from pyscf import M
# assuming the same O-H length for the two bonds
def get_h2o_m(bond_angle, bond_length):
phi = bond_angle / 2
r = bond_length
O = ["O", 0, 0, 0]
H1 = ["H", -r * np.sin(phi), r * np.cos(phi), 0]
H2 = ["H", r * np.sin(phi), r * np.cos(phi), 0]
return M(atom=[O, H1, H2])
[2]:
# PES range
bond_angles = np.linspace(80, 115, 10) / 180 * np.pi
# in angstrom
bond_lengths = np.linspace(0.90, 1.15, 10)
# build molecules
from itertools import product
moles = []
for bond_angle, bond_length in product(bond_angles, bond_lengths):
moles.append(get_h2o_m(bond_angle, bond_length))
len(moles)
[2]:
100
Calculate¶
For each mole in moles, calculate UCCSD energy using the UCCSD class from TenCirChem.
[3]:
import time
from tencirchem import UCCSD
res = []
time1 = time.time()
for m in moles:
# skip CCSD and FCI to speedup
uccsd = UCCSD(m, run_ccsd=False, run_fci=False)
res.append(uccsd.kernel())
time2 = time.time()
time2 - time1
[3]:
38.372753381729126
Plot¶
[4]:
from matplotlib import pyplot as plt
plt.contourf(bond_lengths, bond_angles / np.pi * 180, np.array(res).reshape(10, 10), cmap="Blues_r", levels=50)
plt.colorbar()
plt.xlabel("bond length (Å)")
plt.ylabel("bond angle (°)")
plt.show()
[ ]: